
If you’ve ever been curious about how electric car batteries work, this is the article for you. From their construction to usage, this guide will give you all the details about how these batteries function and help your electric car knowledge go further than ever before. Electric car batteries are typically made up of many individual cells connected in series and parallel to form a battery pack. These cells use a chemical reaction to store and release electrical energy. Simplified Internal Mechanism of Electric Automobile Batteries
The internal working of the electric car battery involves the chemical reactions happening at the time of the charging and the discharging. There are various types of electric vehicle batteries that are composed differently and with different materials. The most common type of electric car battery is the lithium-ion battery. Inside each cell, there are two electrodes – a positively charged cathode and a negatively charged anode.
Here we discuss what is the internal working of the electric car battery. The types of electric car battery, the structure of the electric car battery, and how an electric car battery work.
[toc]
Types of electric car battery
When it comes to electric car batteries, the most popular name is lithium-ion batteries. This is because that’s what the automakers use in their cars. While being the most popular type, lithium-ion batteries are also made to the news of their higher incidence of fires.
The electric vehicle battery is considered a lithium-ion battery, But there are other types of batteries too. So here are the various types of electric car batteries used in electric vehicles.
- Lead Acid Battery
- Nickel Cadmium Battery
- Nickel Metal Hydride Battery
- Lithium Ion Battery
- Solid State Batteries

Lead Acid Battery
Though lithium-ion batteries are dominating, lead-acid batteries are still in use. They are not used in traction anymore, but they are used to power the circuit of electric cars. Lead acid batteries are bulky, and provide less power than that lithium-ion batteries. But they are inexpensive and easy to produce. Lead acid batteries are not as hazardous as lithium-ion batteries. And are easy to recycle.

Electric cars use lithium-ion batteries and lead-acid batteries. Lithium-ion batteries are responsible for all that luxury and most of the features. While lead acid batteries power the basic circuit of the car.
Lead acid batteries have been used in automobiles for decades, since the 1980s. With time they got replaced by more efficient technologies. Manufacturers and companies are developing newer versions of these batteries with more capacity.
Types of Lead Acid Battery
In a broad way, lead acid batteries can be said to be classified into these 2 types.
1. Flooded Lead-Acid Battery

These batteries are said to be Flooded Lead Acid Batteries because the electrodes made up of lead and lead oxide are dipped into the sulfuric acid. We will discuss it later in this article. The thing here to know is that these batteries are used in car start batteries, ATV batteries, Golf cart batteries, motorcycles, and even solar backup systems.
2. Sealed Lead-Acid Batteries (SLA) Or Valve-Regulated Batteries (VRLA)

Another type of lead acid battery talked about is the Sealed Lead Acid Battery which is also called the VRLA means Valve regulated Lead Acid Battery.
Sealed Lead Acid (SLA) batteries are lead acid batteries with a sealed case, so as to prevent the escape of hydrogen, oxygen gas, and water vapors. These gases are forced back into the battery to recombine and form water, and there is no need to add water to the battery to function. Sealed Lead Acid Batteries (SLA) batteries can be found being used in deep cycle operations and engine starts.
Valve Regulated Lead Acid Batteries (VRLA) batteries are also a type of Sealed battery. In Valve-regulated Lead Acid (VRLA) batteries, the escape of the hydrogen and oxygen gas is regulated via the valve in order to escape them safely. These types of batteries are found in toys, medical scooters, and alarms.
AGM batteries can be said to be another variation of sealed electric car batteries. Absorbing glass mat (AGM) structure involves electrolytes suspended close to the plate’s active material. They can be found to be used in RV batteries and ATV batteries.
Gel batteries are similar to AGM lead acid batteries, the difference is that they contain silica additives which solidify the electrolytes. They are used in wheelchairs and RV deep-cycle batteries.
Lead acid batteries are probably the longest-used batteries, and the automobile sector can’t deny their importance. Over the years several efficient technologies came and replaced it in various functions. Still, lithium-ion batteries are now being used in electric cars as secondary energy storage sources.
Nickel Cadmium Battery
Nickel Cadmium (Ni-cd) Batteries were used in electric vehicles in the 90s. But Nickel Cadmium Batteries are prohibited in electric vehicles due to the toxicity of the cadmium, says the renault group.
Nickel-cadmium (Ni-cd) batteries also abbreviated as Ni-Cd batteries, have a lot of advantages. They have a higher power storage capacity, are more stable, and have a higher lifespan. Nickel-cadmium batteries can continue up to 500 to 1000 charging cycles.
A paper from 1995 predicted that between 1995 to 2005 Nickel Cadmium batteries may account for anywhere between 20 to 50 percent of the electric vehicle battery market.
Here is a graph from mdpi about how the demand for the different types of electric vehicle batteries is changed over the years. Nickel Cadmium batteries have declined in this, due to the toxicity of the cadmium.
Nickel Cadmium batteries can be found to be used in laptops, camcorders, and other rechargeable types of gadgets.
Nickel Metal Hydride (NiMH) Battery

Nickel Metal Hydride batteries being used in hybrid electric vehicles (HEVs) are different front he lithium-ion batteries of course. Nickel Metal Hydride batteries have a longer lifespan than that of lithium-ion batteries.
Going into the internal structure, the basic difference between lithium-ion batteries and nickel metal hydride batteries is power storage. In Lithium-ion batteries carbon and lithium which store more energy than the other materials. While in the Nickel- metal hydride batteries hydrogen along with titanium is used to store power along with other metals.
The other thing to know is that they don’t get power from an external source, that’s why they are less useful in plug-in electric vehicles. And thus they are used in hybrid electric vehicles more. The weaker side of nickel metal hydride batteries is that they have a higher discharge rate, heat more, and are more expensive.
Nickel metal hydride batteries are better accepted as they don’t contain harmful and toxic heavy metals which was a problem with nickel-cadmium batteries. The Nickel-cadmium batteries were most economical in the 2000s until the lithium-ion batteries were invented, as mentioned in a Renault group blog.
Solid State Battery

Solid-state batteries are getting discussed because lithium-ion batteries have some disadvantages that solid-state batteries don’t have. There are several reasons why solid-state batteries are better than lithium-ion batteries.
Basically, solid-state batteries contain solid ingredients which completely replace the liquid and heavy electrolytes in lithium-ion batteries. The concept of the solid state is, liquid electrolytes with the help of solid ingredients can take the form of polymers or powders. These solid electrolytes make it more compact. The bulkiness of lithium-ion batteries can be solved by solid-state batteries. Solid-state batteries are new to the automobile market and in the electric vehicle market.
Solid-state batteries have a long way to go, and lithium-ion batteries are also getting new and new innovations. In the future, solid-state batteries might come into the large picture.
Lithium-ion Battery

Coming to the main player, lithium-ion batteries have dominated the electric vehicle battery market. The basic internal structure of a lithium-ion electric vehicle battery is that it contains an anode, cathode, separator, electrolytes, and sealing. Well, there is a lot to discuss on the lithium-ion electric vehicle battery internal structure.
The lithium-ion batteries are good when comes to power storage, discharge rate, and performance. They have higher efficiency than other types of batteries and low self-discharge rates. And thermal control is also better.
Properties of the lithium-ion battery
Lithium-ion batteries provide good storage density way more than other electric car batteries. The lithium-ion battery offers 300 to 500 kWh density, which is 10 times more than that of the lead acid battery.
These batteries are more efficient than lead-acid batteries. Lithium-ion batteries are durable. They are fast charging. The voltage provided by lithium-ion batteries is better than the other batteries. The lifespan of lithium-ion batteries is longer. The lithium-ion batteries are the best choice for automakers for electric vehicles.
Despite these advantages, lithium-ion batteries have become a matter of concern, due to 2 reasons.
One is that they catch fire.
The fire caused by lithium-ion batteries is extremely hard to extinguish, as it contains hazardous vapors too. The individual lithium cells keep catching fires even if you extinguish them.
Another concern is disposal and recycling.
Millions of electric vehicles are using lithium-ion batteries. All these lithium-ion batteries when die, what will happen to the old electric vehicle batteries?
The leaching causes the soaking of harmful chemicals into the ground, surface waters, and soil. This ultimately leads to detrimental effects on the ecosystem and its components. Animals, humans, and plants get severely affected by the improper disposal of old electric car batteries. The electric car lithium-ion batteries eventually get corroded.
Disposal of lithium-ion batteries is harmful, as they leach into landfills and may harm the environment. Recycling is an option. Recycling is expensive and complicated. But for now, they are getting recycled by automakers and recycling firms.
Why Lithium-ion Batteries only?
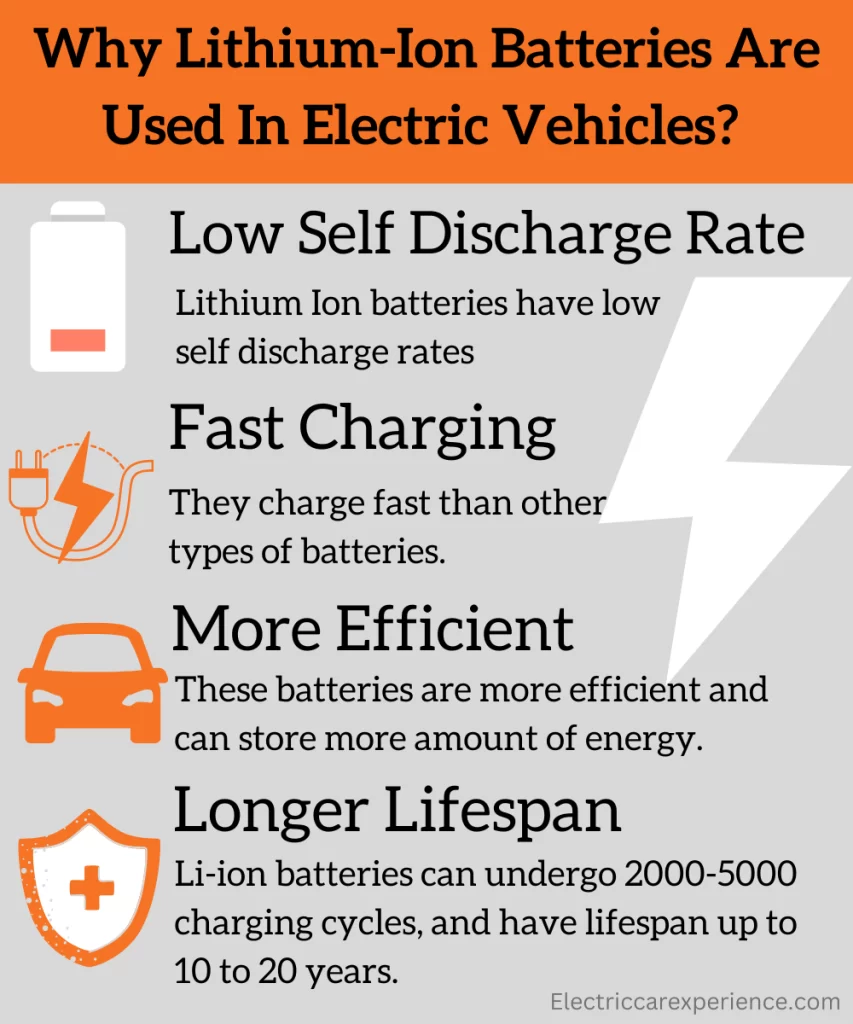
You might develop a question in your mind that why lithium-ion batteries are mostly used in electric cars and not other types of batteries. The reason is simple. At this point in time, the lithium-ion battery seems to be an efficient as well as economical combination of good energy density, efficiency, cost, weight, integrity, low self-discharge rates, and fast charging.
The lithium-ion batteries are more efficient than other types of batteries. It has a low self-discharge rate and also doesn’t have a memory effect. Lithium-ion batteries can undergo 2000 to 5000 charging cycles. The lithium-ion batteries can last about 10 to 20 years, so they have a long lifespan.
Due to all the above reasons, Lithium-ion batteries are used in electric vehicles rather than the other types of electric car batteries.
Battery Construction | Internal Structure of the Electric Car Battery
Here is a lot to discuss. As there are various types of electric vehicle batteries. It is important to know the construction of electric vehicle batteries in order to know the internal working of the electric car battery.
Structure of the Lithium-ion battery cell used in Electric vehicles
The basic structure of the lithium-ion battery consists of an anode, cathode, electrolytes, and separator, like most chemical batteries.
The Lithium-ion batteries in electric vehicles, contain individual lithium-ion cells arranged and connected to each other in a circuit. Based on the number and arrangement of these individual lithium-ion cells, the capacity, and energy density of the battery are dependent.
So coming to the basic functional unit of the lithium-ion battery is the lithium-ion cell. Lithium-ion cell is composed of the Anode, cathode, electrolytes, and separator.
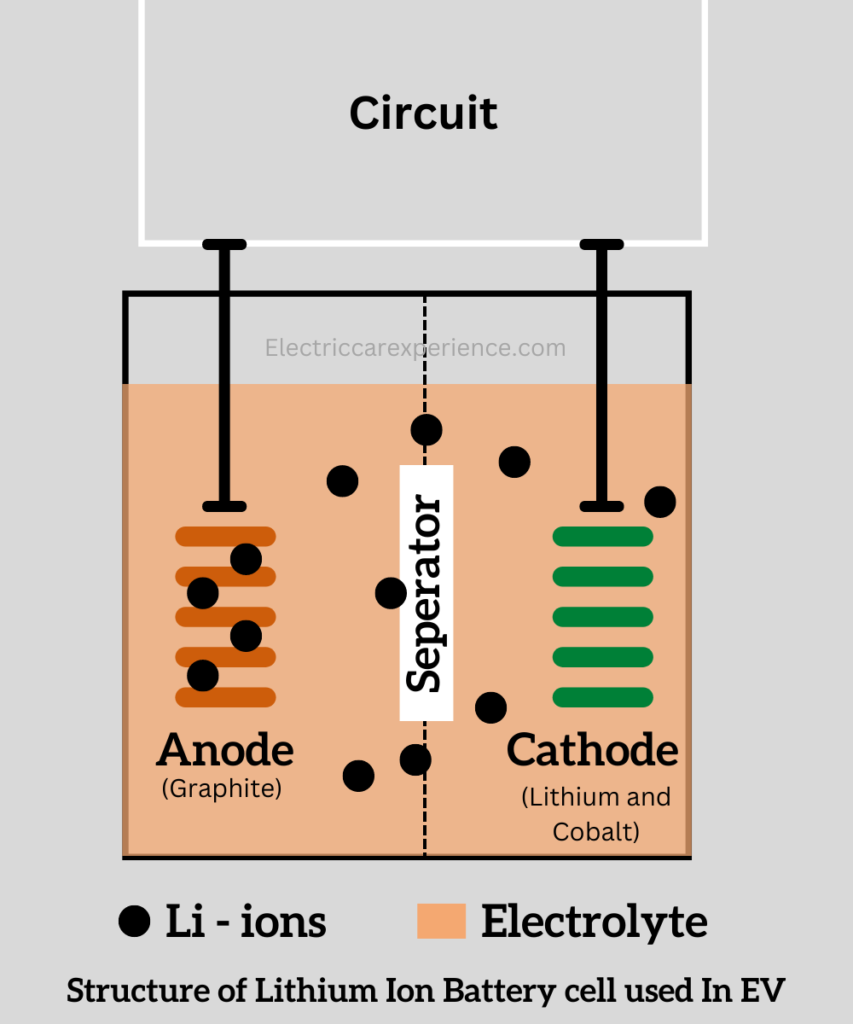
Anode:
The anode in the lithium-ion battery is made up of graphite usually. The graphite got a layered structure that can store the lithium ions in between.
The anode is an important part of the lithium-ion battery. At the time of charging, the anode store the electrons, and at the time of the discharge releases them. We are going to discuss this in the section called “internal working of the electric car battery”.
Cathode
The cathode is the equally important complementing part to the anode, in lithium-ion batteries. In a lithium-ion battery cell, the cathode is made up of lithium and cobalt, or lithium cobalt oxide. Lithium is not stable as an element, that’s why cobalt is used along with it.
The importance of the cathode increases because the amount of lithium in it determines the energy density of the lithium-ion battery. The cathode is positively charged and helps in the charging and discharging process and flow of the lithium ions. The lithium ions can get released from the cathode and intercalated into the anode. The cathode undergoes oxidation at the time of the charging.
Electrolytes

Electrolytes are the liquids that actually make the flow of ions from one electrode to another. Both anode and cathode are immersed in the electrolytes. The electrolytes are the medium in which the electrons, and ions travel. The electrolytes are the lithium salts dissolved in the organic solvents.
There is a variety of organic solvents that can be used. Here we went through some battery structures and research papers and here are the solvents that are used as the electrolytes in lithium-ion batteries.
The typical electrolyte for LiBs is made of a flammable carbonate-based organic solvent such as ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC), and/or propylene carbonate (PC) , with additives including lithium hexafluorophosphate (LiFP6), lithium hexafluoroarsenate monohydrate (LiAsF6), lithium perchlorate (LiClO4), and lithium tetrafluoroborate (LiBF4) to improve cycling .
–Review of lithium-ion battery fire suppression.
Electrolytes are being researched and are a matter of concern because they are the flammable ingredient in lithium-ion batteries. The electrolytes can catch fire, That’s why RTIL, Room Temperature Ionic Liquids are being researched, to be used as the solvent in the electrolytes.
Separator
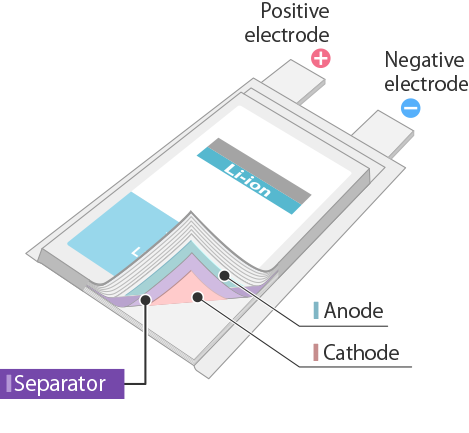
The separator is the physical barrier that prevents the direct flow of the electrons of the anode and cathode. It keeps the anode and cathode separate and, allows only ions to pass through it.
The separator can be said partially permeable as they only allow the ions only. The separator in lithium-ion batteries is made up of permeable polymer membranes, which are usually Poly ethylenes or Poly-propylene. The separator is an important factor in the safety of a lithium-ion battery.
The separator is an essential and extremely important component of the lithium-ion battery. To perform the internal working of the electric car battery and to make it safe, the separator is important.
The function of the operator is to prevent direct contact between the anode and cathode. This prevents sudden short-circuit and thus makes the working of the battery safe. In case of accidents, if the separator is damaged, student fire can cause. So a separator is important.
All these components, anode, cathode, separator, and electrolytes make up one lithium-ion unit of the lithium-ion battery. These individual lithium-ion cells are arranged and packed in order to function as a battery. Other than these components the lithium-ion battery is also connected to a dedicated circuit in the electric vehicle.
This is how electric vehicle batteries are packed. | Different stages of the electric vehicle battery structure
The electric vehicle battery is a bulky, heavy battery pack that fits into the vehicles. But Do you know how it forms? The small units of the battery combine to form the electric vehicle battery pack and that’s what we see.
And that’s why we can say that the lithium-ion batteries used in electric vehicles are different from normal lithium-ion batteries. The Battery pack of the lithium-ion battery in an electric vehicle can be divided structurally into three stages or parts. And at every stage, the battery is usable and can be used in various applications. The three stages are the Battery cell, battery module, and then battery pack.
The complete structure of the Electric car battery| Structure of the Electric car Lithium Ion Battery
The battery in electric vehicles is not just made up of one lithium-ion cell. Instead, a number of such cells are packed together. . There are various stages of an electric vehicle battery structurally, and at each stage, it can be said to be a battery and can be used for different applications. The electric vehicle battery can be divided into three stages.
- Battery cell
- Battery Module
- Battery Pack

Battery cell
A battery cell is the basic unit of a lithium-ion battery or any other electric vehicle battery. The cells are made up of the anode, cathode, separator, and electrolytes as we discussed above. These all cells can combine further to produce the next stage of electric vehicle batteries.
Battery cells can be said as the first stage of the formation of the battery pack in the electric vehicle. They can be said as the basic functional unit of the battery pack, as same as a cell is to the human body. The Lithium-ion battery cell is made up of the cathode, anode, electrolytes, and separator. That’s what we have just discussed above in the structure of the electric vehicle battery.
Battery module
The battery module is made up of multiple battery cells packed together, arranged in a series or parallel, or both manner, in the same housing frame. Basically, a number of battery cells combine to form a battery module.
In the specifications of the various electric car battery pack, you might have seen the word module. The modules are nothing but a number of battery cells that come together in a specific way. Then these battery modules combine to form the next stage of the battery.
Battery pack
The battery pack is made up of several battery modules packed together along with the BMS and other systems. The battery pack is something we consider a complete Electric car battery. Instead of directly combining the battery cells automakers prefer to combine them into the modules and then combine the modules to form a battery pack.
This is done in order to improve the range and capacity of the battery. And handling modules is way more efficient. This makes the complete structure of the electric vehicle battery pack.
Traditional Vs Structural Battery Pack
Traditional battery packs provide a good frame to the battery and good to provide energy. The Structural battery packs take this to a further level. What is the difference between structural battery packs and traditional battery packs?
In traditional battery packs, the battery cells are enclosed in battery modules. While in the structural battery packs, the cells are bonded together, and then to the upper and lower metal sheets. This provides more strength and structural integrity. Tesla’s structural battery has reduced the weight and improved the structural integrity. The structural battery packs caused significant reductions in the number of parts.
The structural battery pack is part of the vehicle’s chassis, as the battery pack acts as a structural part of the whole car. Structural battery packs are designed well and they provide structure to the vehicle along with storing energy. Tesla’s tabless batteries are also said to be a good option for making structural batteries more well-structured.
While being said more efficient, structural battery packs have been talked about disadvantages. The one disadvantage the structural batteries is said to be that they are not replaceable, if an accident happens they are likely to get totaled. While tesla manual has confirmed that the structural batteries are replaceable, and can be disassembled too.
Currently, Tesla is working on the structural batteries to improve more. Tesla Model Y is the only model which has a structural battery, for now. In the future, we may see more vehicles with lightweight structural batteries.
Here we discussed the structure of the electric car battery, specifically the lithium-ion battery. Now let’s see the Internal working of the electric car battery.
Internal Working of Electric Car Battery
Understanding the Internal working of an electric car battery is simply a matter of a few minutes if one has understood the structure of the battery cell. The internal working of the battery involves chemical reactions such as oxidation and the flow of electrons.
The internal working of an electric car battery can be explained better by explaining the charging and discharging process. The Lithium-ion battery uses the flow of lithium ions to charge or discharge the battery.

Discharging
When the Power of the battery is being used, it undergoes discharging. Oxidation means loss of electrons. During The Discharging, the electrons that are accumulated (stored/ intercalated) in the anode (negative electrode) get released. These electrons enter the circuit and flow to the cathode (positive electrode), and generate electric energy.
Due to the release of electrons, the Li+ ions are formed. Due to the difference in potential, the lithium ions get naturally flow to the cathode. The separator allows only the ions to flow.
Charging
At the time of the charging, the oxidation reaction occurs at the Cathode. In a lithium-ion battery cell, the cathode is made up of lithium and cobalt, or lithium cobalt oxide.
Due to the power given by the charger, the cathode (positive electrode) gets oxidized and loses electrons. These electrons get released and flow back to the anode (negative electrode).
While on the other hand, Lithium ions get absorbed again in the anode during charging. The lithium ions get stored in the anode. We can say that the lithium-ion electric car battery store energy in the form of lithium ions. The amount of lithium ions stored determines the capacity of the battery.
The charging and discharging cycle repeats, and this is how an electric car battery work. Below table shows the difference between charging and discharging and reactions at anode and cathode.
| Charging | Discharging |
|---|---|
| Oxidation reaction occurs at the cathode. | Oxidation Reaction Occurs at the anode. |
| Electrons flow from the anode to the cathode. | Electrons flow from the cathode to the anode. |
| Lithium ions get intercalated in the anode. | Lithium ions get released From Anode. |
| The electric energy is converted to chemical energy. (by the use of the external energy source, the energy is stored in the battery in the form of lithium ions.) | The chemical energy is converted to electric energy. |
| The power from an external sources is taken to store energy. | The stored energy is used to provide power to electric vehicles. |
So basically Internal working of an electric car battery involves charging and discharging.
- When we plug in an electric car, electrons that are accumulated in the anode (negative electrode) get released and they enter the circuit and flow to the cathode (positive electrode), and generate electric energy.
- When the electric vehicle is using the generated power, the cathode (positive electrode) gets oxidized and loses electrons. These electrons get released and flow back to the anode (negative electrode).
This is How Electric Car Batteries Work
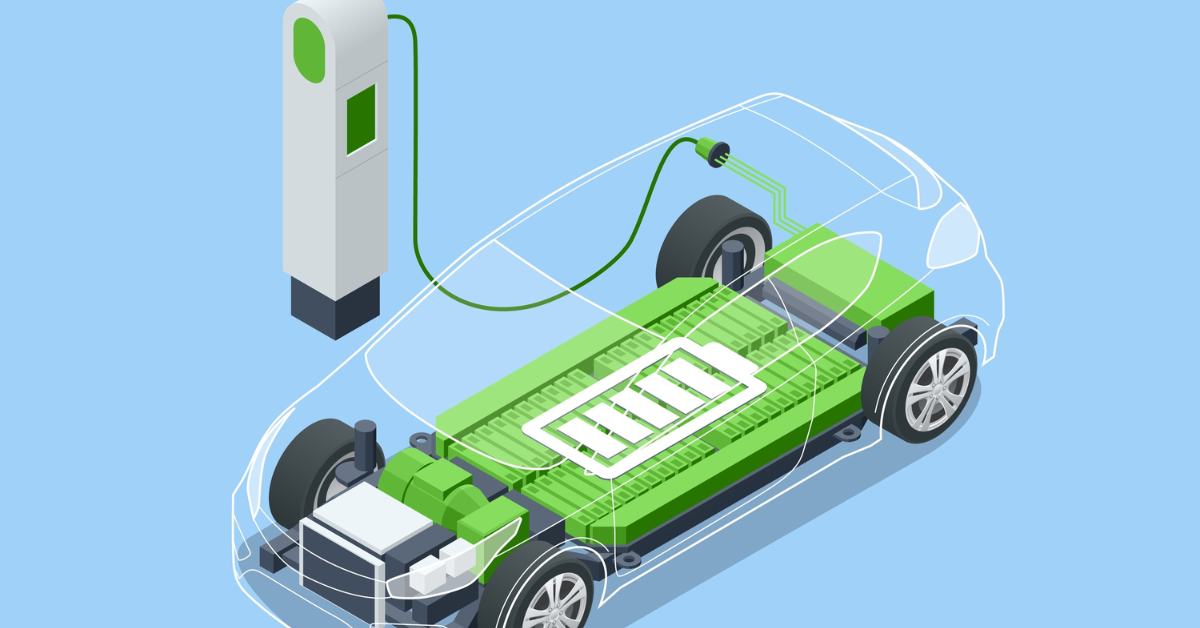
Apart from the chemistry, we have also discussed the internal working of the electric car battery in a broad way. When the electric car needs power, the battery powers the motors of the vehicle. Once the power of the electric car battery is depleted, we need to plug in an electric vehicle to charge the battery.
The battery gets the energy and gets charged by getting power from the electric vehicle grid, and stores the energy in the form of lithium ions. Then at the time of the discharge, the battery again powers the vehicle. This is how electric car batteries work in a broad sense.
The internal working of the electric car battery at the chemical level involves the flow of lithium ions, electrons, and the oxidation reaction. At the time of the charging oxidation occurs at the cathode and the lithium ions get stored in the anode. The more the number of lithium ions stored, the more is the battery charged.
While discharging, the same accumulated lithium ions flow, and the stored energy is utilized. That’s it. The energy can be used by motors, electronics, etc. Of course, the circuit is attached to the battery, in order to convert chemical energy to electric energy. The electrons flow through the wire and the circuit, and this causes the formation of electric energy. This electric energy then powers the motors of the electric vehicle.
Conclusion
Conclusion #1: Electric car Battery Structure
Electric car batteries are a very important part of the vehicle’s operation. They store energy and provide power when needed by the motor or other components inside the vehicle.
The basic structure of the lithium-ion battery consists of an anode, cathode, electrolytes, and separator, like most chemical batteries. The anode is made up of graphite and the cathode is of cobalt and lithium. The charging and discharging are the matter of the flow of the electrons and the lithium ions.
The battery modules give more structural integrity. Then comes the battery pack, which we call as the electric vehicle battery. Tesla is making use of structural batteries and they are said to be better than that traditional battery packs.
Conclusion #2: Electric car battery Stages
The amount of lithium determines the capacity of the battery. The battery can be divided into three stages, the basic is the battery cell. The further stage is the Battery module. Battery module s a combination of battery cells arranged in a series or parallel manner.
Solid-state batteries are also being developed to compensate for the disadvantages of lithium-ion batteries. The basic internal working of the battery is dependent on the flow of the lithium-ion batteries. The charging and discharging cycle runs throughout the life of the battery as long we use the battery.
Conclusion #3: Electric car battery power
The discharging depends on the amount of power the other features of the battery required. After discharge, we have to plug in the charger, and the battery gets power from the grid. This leads to the reverse flow of the electrons and the lithium ions get intercalated into the anode.
This blog post has described how electric car batteries work, but it also made you learn more about what makes lithium-ion batteries unique compared to other types of batteries used for cars.
FAQ
Why lithium-ion batteries are used in electric vehicles?
The lithium-ion batteries are used in electric vehicles because they are more efficient than other types of electric vehicles. They are fast-charging and have longevity.
The lithium-ion batteries last for 10 to 20 years and are way more powerful than conventional lead-acid batteries. Due to their several advantages over other types of batteries, lithium-ion batteries are being used in electric vehicles, dominantly.
What happens at the cathode when the electric car battery is charging?
When the battery is charging, oxidations occur at the cathode. Due to the power given by the charger, the cathode (positive electrode) gets oxidized and loses electrons.
These electrons get released and flow back to the anode (negative electrode). While on the other hand, Lithium ions get absorbed again in the anode during charging. The lithium ions get stored in the anode.
What type of batteries are used in electric vehicles?
The various types of batteries used in electric vehicles are lithium-ion batteries, lead acid batteries, nickel-cadmium batteries, nickel metal hydride batteries, and solid-state batteries. From that, lithium-ion batteries are mostly used and can be found in most electric vehicles. While the nickel-cadmium battery is prohibited because of the toxicity of cadmium.
How does an electric car battery work?
Basically, electric car battery work by charging and discharging. After plugging in an electric car, electrons that are accumulated in the anode (negative electrode) get released.
These electrons enter the circuit and flow to the cathode (positive electrode), and generate electric energy. While the electric vehicle is using the generated power, the cathode (positive electrode) gets oxidized and loses electrons.
These electrons get released and flow back to the anode (negative electrode). The charging and discharging cycle continues as long as the battery is being used.
How long does an electric car battery last?
Electric car batteries last 10 to 20 years. It depends on the electric vehicle automakers, the type of the battery, the condition of the battery, and the use of the battery.
If the battery has a higher capacity it can travel long range while other batteries cannot. Automakers warranty the electric vehicle for 5 to 8 years for the electric car battery replacement. However, with proper maintenance, electric car batteries can last up to 10 to 20 years.
Post Related to Electric Car And Battery
- Why Tesla owner locked out of the car?-A complete guide 2024
- Does Radio Drain The Electric Car Battery?
- Can I Put A Bigger Battery In My Electric Car?
- How Cold Is Too Cold For Tesla Battery?
- Can a swollen car battery be fixed?
- Cheapest Electric Car Battery Replacement Cost
- Tesla Model s original battery condition after 10-year usage (20-year usage, 5-year usage, 2-year usage)
- Can Tesla Put More Batteries In The Front Trunk Of The Tesla Model S To Improve Range?
- Why China is working hard to control the resources used to make batteries for electric cars? An ultimate guide 2024
- What Is The Duration Of Kia Electric Car Battery Warranty?
- Can’t Electric Cars Charge A Spare Battery and Replace It When The Main Battery is Over?
- How To Charge A Leisure Battery And How Long To Charge?
